| January, 1996 Volume 19, Issue 1 Dave’s Preferred Priming
Procedure
Reprinted by permission from
Dave Draper
david.draper@mq.edu.au
http://www.ocs.mq.edu.au/~ddraper
Introduction
Most brewing
textbooks instruct brewers to prime their beers with an
amount of priming sugar on a volume per volume basis. The
level most commonly cited is 3/4 of a cup per 5 (US)
gallons. This can give rise to problems, however: not all
sugars are manufactured in the same way, so that 3/4 cup
of one type might in fact be a very different amount than
3/4 cup of another type. It is not clear whether it
should be a packed cup, a loose cup, or what. It seems
extremely obvious to me (and to some others I've talked
with) that a better way is to weigh the amount of sugar
being used, and prime on the basis of weight sugar per
volume beer--for example, grams per litre (g/L). This
document is intended to show how this can be done in
practice, and why it is a superior method. I acknowledge
the helpful input from the following net.brewers: Mark
Hibberd, who really should be thought of as a
"co-author" of this page--his document on
priming available at The Brewery (latest update soon to
be installed) saved me a lot of legwork; John DeCarlo,
whose question motivated the water-adsorption tests
described below; and Dick Dunn, who provided additional
evidence of how different sugars might compact (he
reports seeing dextrose/glucose compact up to 30% with
just a gentle tap!).
Now, it is of
course true that if we proceed in the same way every
time, we will eventually arrive at a set of amounts that
work for us. However, in order to reduce the
trial-and-error aspect as much as possible, it would be
desirable to start more or less from first principles so
that we can have a better idea what we are doing,
particularly when entering as-yet-uncharted territory.
Part 1.
Arriving at the desired priming rates.
First a brief
rundown of the main idea: carbonation is most usefully
described in terms of volumes of CO2. A beer
carbonated to 2 volumes would have, say, 2 litres of CO2
in every litre of beer. See Dave Miller's books for a
more complete discussion. Mark Hibberd's priming guide
gives the following handy guide to carbonation levels in
a range of styles:
Beer Style
|
Volumes CO2
|
British-Style Ales
|
1.5 - 2.0
|
Porter, Stout
|
1.7 - 2.3
|
Belgian Ales
|
1.9 - 2.4
|
European Lagers
|
2.2 - 2.7
|
American Ales & Lagers
|
2.2 - 2.7
|
Lambic
|
2.4 - 2.8
|
Fruit Lambic
|
3.0 - 4.5
|
German Wheat Beer
|
3.3 - 4.5
|
Mark's guide
also shows that beer that is ready to bottle, having had
CO2 bubbling through it more or less
continuously, will be CO2 saturated, and that
the amount of CO2 dissolved is a function of
the temperature of the beer. At lower temperature, the
beer can dissolve more CO2. Accordingly, we must take
this into account, and prime enough only to add the
appropriate number of volumes to that already present,
thereby arriving at our desired value.
Here is a
list of the saturation values from Mark's paper. These
numbers represent how many volumes of CO2 are
in the beer at the listed temperatures before we add any
priming sugar:
Temp. ºC (ºF)
|
Volumes CO2
|
0 (32)
|
1.7
|
2 (35.6)
|
1.6
|
4 (39.2)
|
1.5
|
6 (42.8)
|
1.4
|
8 (46.4)
|
1.3
|
10 (50)
|
1.2
|
12 (53.6)
|
1.12
|
14 (57.2)
|
1.05
|
16 (60.8)
|
0.99
|
18 (64.4)
|
0.93
|
20 (68)
|
0.88
|
22 (71.6)
|
0.83
|
The reaction
that produces CO2 during carbonation is one in
which one mole of glucose, C6H12O6,
goes to 2 moles of ethanol, CH3CH2OH,
and 2 moles of CO2. A little stoichiometric
algebra shows that we will add 1 volume of CO2
for every 3.7 g/L glucose added to the beer. So now that
we are armed with the temperature dependence data and the
amounts from this reaction, we can produce a general
predictive relationship to use in our brewery.
The plot
below shows how many volumes of CO2 will be
produced in the finished beer by priming at the level on
the x-axis. Each line is labeled for the temperature of
the beer being primed, and incorporates the amount of CO2
present prior to priming. We choose the carbonation level
we desire, then find the line that corresponds to the
beer's temperature, and finally read off the g/L priming
rate that will give the desired carbonation.
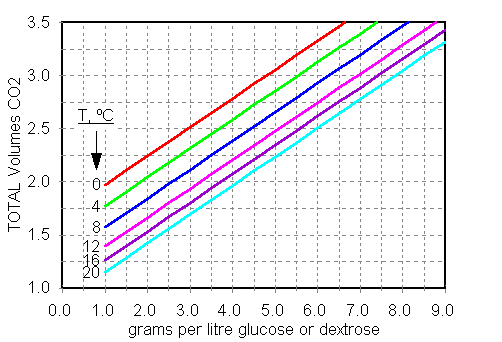
Example 1. We
have a lager at 4°C and want it carbonated at 2.75
volumes. We find 2.75 on the y- axis, then move over
until we hit the 4°C line, then read down to get about
4.5 g/L. Note that if this beer were at 20°C, we would
have to prime at about 7 g/L to get the same level of
carbonation, because of the lesser amount of CO2
in the beer before priming at that higher temperature.
Example 2. We
have a pub bitter at 16°C that we want carbonated at 2
volumes. This time it requires about 3.7 g/L.
The lines on
the plot above can be expressed as equations as well. To
calculate the priming rate in g/L, first find (from
Mark's table above) the saturation level at the
temperature of the beer--let's call it v0.
Then choose the volumes of CO2 that correspond
to the desired carbonation level--let's call that v.
Then
v - v0
Rate in g/L =
0.27027
You can
confirm this using the two examples given above. For
Example 1, the expression gives (with v0
= 1.5 at 4°C and v = 2.75) 4.6 g/L, and for
Example 2 (v0 = 0.99 at 16°C, v
= 2) we have 3.7 g/L.
If one is
priming with sucrose, i.e. table sugar or brown sugar, it
turns out that about 20% more CO2 is produced
per g/L priming. I will not reproduce the graph for this
case--it's just like the one above except the lines are
displaced. The corresponding equation to use if priming
with sucrose is:
v - v0
Rate in g/L =
0.33784
Conversions
of all this to screwed-up-British-engineering units is
left as an exercise for the reader; but the conversion
from g/L to dry ounces per US gallon is:
1 g/L = 0.133
oz/US gallon
See why the
metric system is better? :-}
Part 2.
Testing the reliability of volume vs. weight measurements
John DeCarlo
raised the important question that perhaps, because sugar
can adsorb water vapor from the air and thus increase its
weight, volume might be more reliable a measure than
weight. To test this, I did a simple experiment.
First I
placed several grams of three types of sugar in open
containers for a couple of weeks, so that it could adsorb
as much water as possible. I did this with brewing
dextrose, plain white table sugar, and brown sugar. The
white and brown sugars are both sucrose, of course. Then,
I placed the vials of sugar on a hot plate set at 80°C
for 24 hours to drive off the adsorbed water. I at first
tried to use a drying oven set at 110°C, but this is
above the melting point of dextrose, so I was forced to
use the hot plate. I took no special steps to ensure that
the sugar was totally dry before being exposed to the
air, because this most closely mimics the situation for
most brewers; and it provides a worst-case result, which
is what I am after here.
The results,
tabulated below, suggest that the amount of water uptake
was negligible, assuming that 24 hr at 80°C is
sufficient to drive it off. The amounts ranged from 0.05%
by weight for white sugar to 1.2% by weight for dextrose.
I conclude from this that the uncertainty on the weight
of sugar from adsorbed water is well within the noise of
the types of scales used by most homebrewers.
Here are the
data from this experiment:
Sugar Type:
|
Brown
|
White
|
Dextrose
|
Weight of sugar at start, g
|
1.930
|
3.797
|
2.706
|
Weight after 24 hours, g
|
1.922
|
3.795
|
2.673
|
Percent weight loss
|
0.40
|
0.05
|
1.22
|
Conclusions
1. Because
the effect of carbonation is so important to the overall
impression of a beer, it does not make sense to take a
chance on having the carbonation come out other than
desired.
2. The
governing relations for determining how carbonated a beer
will be, as a function of the weight of priming sugar
used per unit volume, are known and easy to use.
3.
Accordingly, we can control very accurately the
carbonation level of our beers, once we have a feeling
for what a given number of volumes of CO2
''feels like''.
4. There do
not appear to be any problems with using weights as a
result of adsorption of water by sugar. The amount of
adsorption found in a simple experiment was trivial.
|