|
September, 1995 Volume 18,
Issue 9 Chill Out! Part 2 - Thermodynamics Reprinted by permission from Charlie Scandrett (Part 1, August, 1995, dealt with terminology.) I am constructing a 70 litre test model of a full mini-brewery for assembly in central Russia. To this end I have been pestering engineers, beer in hand. FAQ - How do I chill it? With a heat exchanger of course. All our contraptions of copper, plastic and water are heat exchangers. Heat exchanging is a highly advanced industry that knows what it is doing, so why do we reinvent the wheel? The most common classes of heat exchangers are "parallel flow" in which the two fluids (or gases) flow parallel and in the same direction. "Crossflow" in which they flow across each other's path, usually 90 degrees. "Counterflow" is parallel flow in opposite direction. The reason for this terminology is that different mathematical formulas are used to calculate the heat transfer in these different configurations. Heat exchangers are named by "how the two fluids move in relation to each other". The "copper-tube-in-a-hose" is a counterflow heat exchanger. The coolant water should run opposite to the wort because this is by far the most efficient. It is possible to cool the wort to below the exit temperature of the water. The "copper-tube-in-a-bucket / bathtub-of-ice-water" is a crossflow exchanger. It should be continuously stirred and because the flow is not parallel to the copper wall, boundary layers are not a problem outside the tube. The "cold-coil-in-wort" system is also crossflow. The "kettle-in-a-snow-drift" (my Russian grandmother-in-law’s method) is a non-flow system and fraught with temperature stratification problems and has the transmission coefficient of water. (Stir vigorously and it has the same properties as crossflow.) FAQ - Which is best? Well, let us look at some of the basics. Heat must flow from wort to coolant. The main barrier is the metal wall. Wrong! The first trap I fell into was confusing "coefficients of heat transfer" with "coefficients of heat transmission". Heat travels through things and most high school kids could find out that copper is 7 times better at this than steel. But the heat transmission properties of water are lousy and in a laminar flow situation a boundary layer forms on the wall of you tube, becoming an insulator. As well, the interface between liquid/metal and then metal/liquid has it's own resistance called the coefficient of heat transfer. The actual bit of metal (if its under 3 mm) contributes much less than 10% to the "overall coefficient of heat transfer". The rate of heat transfer depends on a few vital factors:
Without trying to understand Prandtl and Renyolds numbers, turbulent flow is achieved in a 1/2" tube at 0.11 m/sec, in a 3/8" tube at 0.14 m/sec, and in a 1/4" tube at 0.22 m/sec. Which quite simply means in each case, "put 5 gallons through your (1/2":3/8":1/4") exchanger in under (22:31:46) minutes and you have turbulent flow", (Charlie's Law) The overall coefficient of transfer jumps from about 1.25 kW/m2 K to about 12.5 kW/m2 K ! Fast flow systems rule, OK? Example: Now 5 gallons of wort at 100 § C cooled to 20 § C needs to lose about 6300 kJ of energy. If the temp difference (D T1) at exit of coolant and entrance of wort (same end) is 30 § C (run the hose fast) and at wort exit (D T2) = 5 § C then the log mean temp difference Q = (D T1-D T2) / (ln (D T1/D T2)) = 13.8 § C.
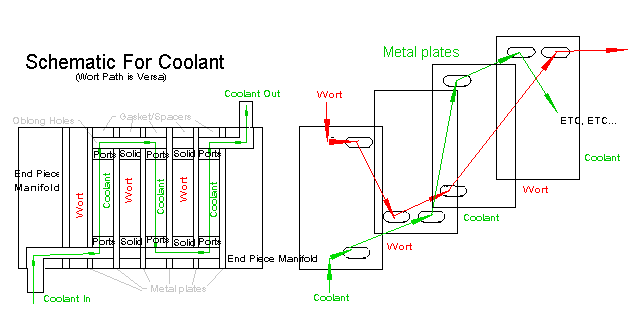
Now Q = U A Q . (Q = heat transfer rate, U = coefficient, A= area, Q = log mean temp difference) If we want to cool the same mass in 10 minutes we need a rate of 10.5 kW which translates theoretically into about 8 feet of 3/8" tube-in-a-hose. Extra area in heat exchanges can't hurt and gives more control, so buy 12 feet. But tap water is seldom 5 § C below the desired final temperature, especially for lager brewers. A crossflow "finishing" exchanger of 4 feet of tube in a ice bucket will pull it down a further 8 > 12 § C without need to change the ice coolant. The "tap-water-in-a-hose-around-a-tube-of-wort" and "tap-water-in-a-coil-in-stirred-wort" are thermodynamically efficient, but suffer from the limitation of the tap temperature as some thermal pressure is needed to drive the heat exchange. The "coil-in-an-ice-bucket / bathtub" systems on their own need coolant changes and constant stirring. The combination counterflow, crossflow exchanger avoids most of this except the stirring. The tap water receives most of the energy, preventing the rapid heating of the ice bucket. To control your cooling for DMS results, the siphon height or outlet constriction will adjust your flow rate of wort and thus your cooling time. The tap water flow rate will adjust your final temperature to the desired figure. Record your results and taste the difference. FAQ - How do I clean it? Simple, a fishing sinker and some 40 lb. line. Drop it down your tube propelled by water pressure and then pull through a rag with whatever cleaning agent you prefer. Those with military training with recognize the rifle pull-through technology. Pubs force tennis-size foam balls through their beer lines under pressure. Not so FAQ - What is a Plate exchanger? If anyone wants to build a simple plate exchanger, please post me. Plate exchangers have coolant and wort flowing on alternate sides of many plates of metal with gasket spacers, a sort of thermodynamic sandwich. Their advantages are massive surface area, good turbulent flow and you can take them apart to clean them! They are run counterflow and can be configured as very efficient heat-recovery pastuerizers.
|
|
|
||